We evolved in an atmosphere that was approximately 21% oxygen and 0.28% carbon dioxide (the balance is inert nitrogen and argon at about 78% and 0.9% respectively, and various trace gasses). But the industrial revolution has altered the atmosphere significantly, and the average concentration of carbon dioxide in air is currently up to 0.042%, an increase of nearly 50% in the last 100 years, and this is projected to rise exponentially over the next several decades. This change is detrimental not only to the earth (causing anthropomorphic climate change) but to the organisms exposed to that climate change. Every living thing on the planet is essentially experiencing respiratory stress in the form of increased CO2.
How do we cope with this? Sensors in the brain respond to pH levels in the blood, which are influenced directly by carbon dioxide. Increased carbon dioxide lowers the pH of blood, and the parts of the brain that regulate breathing respond by instigating faster and deeper breathing (respiratory compensation). The kidneys also play an important role in regulating the pH of blood through the excretion of acids in the urine and the production of bicarbonate, which increases blood pH.
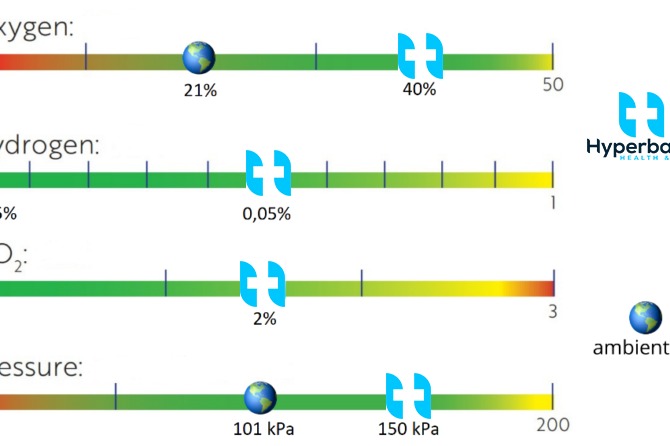
The functional and regulatory components of the respiratory system, then, work together to ensure adequate oxygenation of cells in every part of the body and, within limits, can even compensate for deleterious changes in the atmosphere. But many cells (particularly those at the extreme periphery of capillaries or those with compromised circulation) are under stress from a combination of factors including low oxygen tension, high carbon dioxide levels and free radical by-products of metabolism. The mixture of gasses in the hyperbaric chamber is designed to address these issues by optimizing gas exchange in hemoglobin, increasing dissolved oxygen in plasma, and scavenging free radicals.
Pollutants in the atmosphere also place us under environmental stress. One of the most common atmospheric pollutants is carbon monoxide (CO). The concentration of this toxin in the atmosphere is vanishingly small except in the presence of motor vehicles; average CO levels within 20 meters of an interstate highway can reach levels of more than 4 ppm. Cigarette smoke is another significant source of carbon monoxide – CO in the form of carboxyhemoglobin (COHgb) is present at concentrations of less 1% in non-smokers, whereas smokers have COHgb levels of 5-10%. (This is not detectable by pulse oximeters, which cannot distinguish between heme groups bound to oxygen or carbon monoxide. A patient suffering from fatal carbon monoxide poisoning can have a normal pulse oximeter reading.)
Carbon monoxide exposure accounts for more than half of fatal poisonings worldwide. CO bonds to hemoglobin in the red blood cell with an affinity that is about 250 times that of oxygen, and concurrently increases the stability of oxygen binding to adjacent sites in the heme group so it is less likely to release oxygen in the peripheral tissues. Under standard atmospheric conditions, carbon monoxide binding is irreversible — any heme group that has bound a carbon monoxide molecule is essentially dead. Hyperbaric chambers have long been the treatment of choice for carbon monoxide poisoning in western countries, but more recently, hyperbaric oxygen-hydrogen treatment has been shown to be an effective alternative and even has more advantages (for example, decreased red cell aggregation and reduced free radical production). Hyperbaric chambers with revolutionary Oxygen-Hydrogen treatment seems to be the best available units to support natural healing of many ailments.
More differences and benefits from HBOHT you will find in part 3 of this article.



